THERMODYNAMICS OF MICELLIZATION OF HEXADECYLTRIMETHYLAMMONIUM BROMIDE IN PROPYLENE GLYCOL-WATER MIXTURE: A CONDUCTIVITY STUDY
Abstract
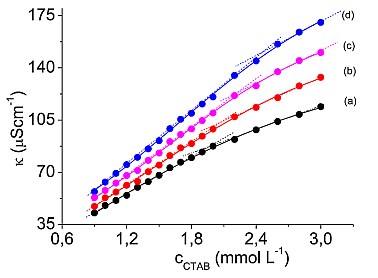
Micellization of hexadecyltrimethylammonium bromide (syn. cetyltrimethylammonium bromide, CTAB) in propylene glycol-water (30% v/v) binary mixture, as well as the thermodynamic properties of the resulting micelles, were investigated by electrical conductivity measurements. The conductivity data were used to determine both the critical micellar concentration (CMC) and the micellar ionization degree (a) of CTAB in the temperature range 298.2-310.2 K. The equilibrium model of micelle formation was applied in order to obtain the thermodynamic parameters (the standard molar Gibbs free energy, DGm0, enthalpy, DHm0 and entropy, DSm0) of the micellization process. The values of DGm0 and DHm0 were found to be negative at all investigated temperatures, while the values of DSm0 were positive and became more positive as temperature increased. A linear dependence between DSm0 and DHm0, i.e. an enthalpy-entropy compensation effect, was observed.
TERMODINAMIKA MICELIZACIJE HEKSADECILTRIMETILAMONIJUM-BROMIDA U SMEŠI PROPILEN-GLIKOL-VODA: KONDUKTOMETRIJSKO ISPITIVANJE
Konduktometrijski je ispitivana micelizacija heksadeciltrimetilamonijum-bromida (sinonim cetiltrimetilamonijum-bromid, CTAB) u binarnoj smeši propilen-glikol-voda (30%, v/v), kao i termodinamičke osobine nastalih micela. Merenjem specifične provodljivosti određeni su kritična micelarna koncentracija (KMK) i stepen jonizacije micele (a) CTAB u opsegu temperatura 298,2-310,2 K određeni su. Primenom ravnotežnog modela za proces micelizacije izračunati su termodinamički parametri: promena standardne molarne Džibsove slobodne energije, (DGm0), entalpije (DHm0) i entropije (DSm0) micelizacije. Vrednosti DGm0 i DHm0 su bile negativne na svakoj od ispitivanih temperatura, dok su vrednosti DSm0 bile pozitivne i povećavale su se sa porastom temperature. Na osnovu linearne zavisnosti između DHm0 i DSm0 utvrđen je tzv. entalpijsko-entropijski kompenzacioni efekat.
Full Text:
PDFRefbacks
- There are currently no refbacks.
ISSN 0354-4656 (print)
ISSN 2406-0879 (online)