AROMATIC Π-NETWORKS IN SM/LSM PROTEIN INTERFACES
Abstract
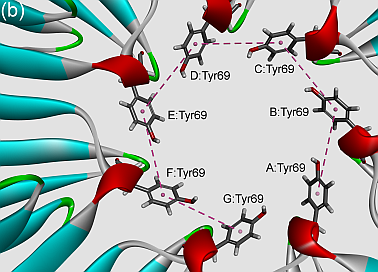
In this work, we have analyzed the influence of π–π interactions on stability and properties of Sm/LSm assemblies. Phe residues were found to be involved in π–π interactions much more frequently than Tyr or His. Similarly, the Phe–Phe π–π interacting pair had the highest frequency of occurrence. Furthermore, a significant number of π-networks were observed at the interface of Sm/LSm proteins. Generally speaking, the distance between the interacting pairs was in the range of 5–6 Å. 3π and 7π-networks were found to frequently have plane-plane angles less than 60º. Solvent accessibility pattern of Sm/LSm proteins revealed that all of the interacting residues were from buried areas. Moreover, most of the π–π interacting residues of Sm/LSm proteins were evolutionary conserved and were in the strand regions. A high percentage of these residues could be considered as stabilization centers that (significantly) contribute to the net stability of Sm/LSm proteins.
AROMATIČNA Π-MREŽA U INTERFEJSIMA SM/LSM PROTEINA
U ovom radu smo analizirali uticaj π–π interakcija na stabilnost i osobine Sm/LSm proteinskih agregata. Ostatak fenilalanina znatno češće uzima učešće u π–π interakcijama u odnosu na His i Tyr. Slično, Phe–Phe π–π interagujući parovi su najučestaliji. Prepoznat je značajan broj π-mreža u interfejsima Sm/LSm proteinima. U većini slučajeva, rastojanje između interagujućih parova aminokiselina bilo je u opsegu 5–6 Å. Za 3π i 7π-mreže, prsten-prsten uglovi manji od 60º su bili učestaliji. Razmatrajući delove Sm/LSm proteina dostupne rastvaraču, može se zaključiti da se svi interagujući parovi nalaze u unutrašnjim regionima. Pored toga, većina π–π interagujućih aminokiselinskih ostataka je evoluciono konzervativan i nalazi se u regionima sa nabranom strukturom. Veliki broj ovih ostataka se može smatrati stabilizacionim centrima, koji (značajno) doprinose ukupnoj stabilnost Sm/LSm proteina.
Full Text:
PDFRefbacks
- There are currently no refbacks.
ISSN 0354-4656 (print)
ISSN 2406-0879 (online)