INVESTIGATION OF REACTION CONDITIONS ON SYNTHESIS OF STEROIDAL BROMOHYDRIN AND STRUCTURAL ANALYSIS OF NOVEL 6α-BROM-5β-HYDROXY DERIVATIVE
Abstract
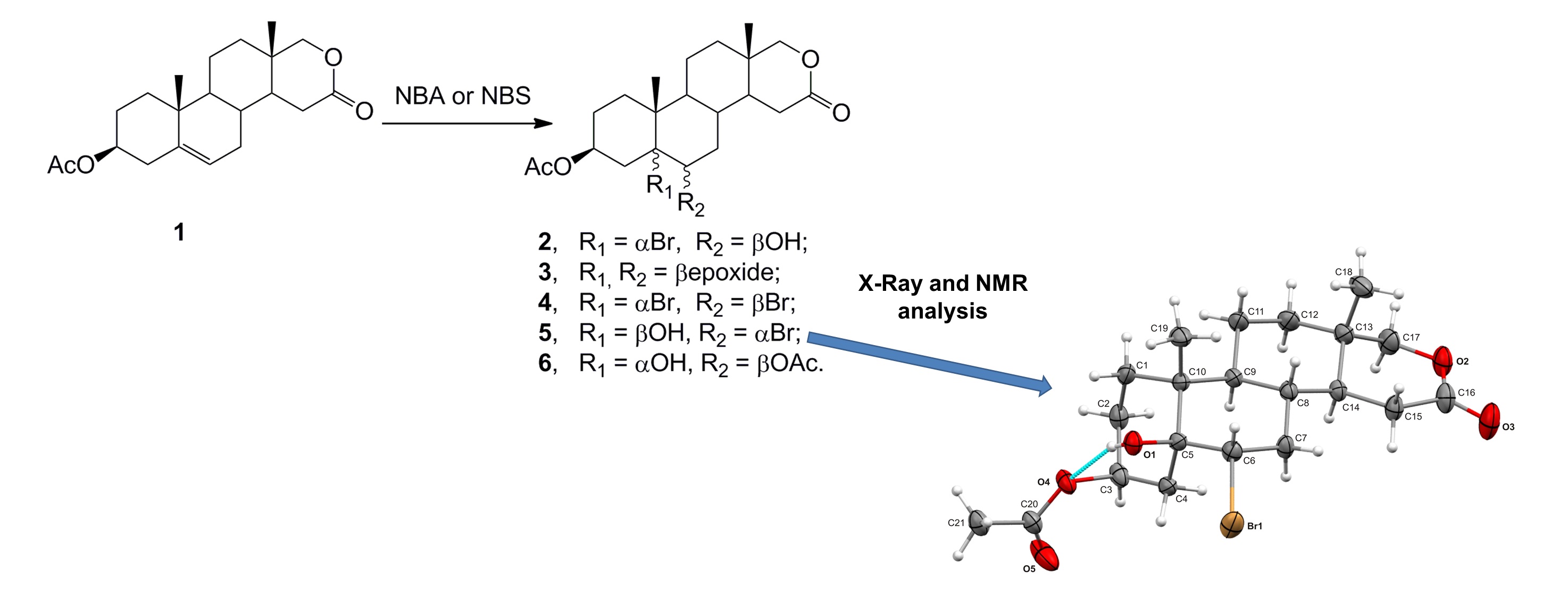
Reaction conditions variation and its influence on the reaction of 3β-acetoxy-17-oxa-17a-homoandrost-5-en-16-one with in situ generated hypobromous acid was investigated. Hypobromous acid was generated from N-bromoacetamide or Nbromosuccinimide and perchloric acid, and as solvent dioxane, dimethoxyethane or tetrahydrofuran were used. After a series of experiments, it was determined that the number of the reaction products depends on the reagent used, solvents, perchloric acid concentration and the presence/absence of daylight. It has also been found that the yields of certain compounds depend also on the reaction time and temperature. 6α-Bromo-5β-hydroxy derivate is obtained by usage of NBA and 0.28 M perchloric acid in dioxane on daylight. Its structure was confirmed by NMR and X-ray crystal structure analysis.
HIGHLIGHTS
- In the reaction of a steroidal alkene with in situ generated hypobromous acid, reaction conditions variation affects number and yields of obtained compounds.
- Most often, 5α-bromo-6β-hydroxy 2, 5β,6β-epoxy 3 and 5α,6β-dibromo 4 compounds are obtained.
- 6α-Bromo-5β-hydroxide 5 and 3β,6β-diacetate 6 are obtained with NBA, 0.28 M HClO4, dioxane and in the presence of daylight at room temperature.
- Configuration on C5 and C6 of compound 5 was determined by NMR and XRay analysis.
Keywords
Full Text:
PDFReferences
Bowers, A., Denot, E., Cuellar Ibanez, L., Cabezas, E. M., Ringold, H. J. 1962. J. Org. Chem. 27, 1862-1867. doi:1021/jo01052a094
Bruno, I. J., Cole, J. C., Edgington, P. R., Kessler, M. K., Macrae, C. F., McCabe, P., Pearson, J., Taylor, R 2002. Acta Crystallogr. B 58, 389-397. doi: 10.1107/S0108768102003324
Clark, R. C., Reid, J. S. 1995. Acta Crystallogr. A 51, 887-897. doi: 10.1107/S0108767395007367
Clark, R. C., Reid, J. S. 1995. Acta. Cryst. A 51, 887-897. doi: 10.1107/S0108767395007367
Farrugia, L. J. 1997. J. Appl. Crystallogr. 30, 565. doi: 10.1107/S0021889897003117
Farrugia, L. J. 1999. J. Appl. Crystallogr. 32, 837–838. doi: 10.1107/S0021889899006020
Grenville, V., Patel, D. K., Petrow, V., Stuart-Webb, I. A., Williamson, D. M. 1957. J. Chem. Soc. 0, 4105-4111. doi: 10.1039/JR9570004105
Gupta, A., Kumar, B. S., Negi, A. S. 2013. J. Steroid Biochem. Mol. Biol. 137, 242-270. doi: 10.1016/j.jsbmb.2013.05.011
Iglesias-Arteaga, M. A., Símuta-Lopez, E. M., Xochihua-Moreno, S., Viñas-Bravo, O., Montiel Smith, S., Meza Reyes, S., Sandoval-Ramírez, J. 2005. J. Braz. Chem. Soc. 16, 381-184. doi: 10.1590/S0103-50532005000300011
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., Forman, D. 2011. Ca-Cancer J. Clin. 61, 69-90. doi:10.3322/caac.20107
Kuzminac, I., Klisurić, O. R., Škorić, D., Jakimov, D., Sakač, M. 2017. Struct. Chem. 28, 567-576. doi: 10.1007/s11224-016-0815-9
Numazawa, M., Yamada, K. 1998. Steroids 63, 62-69. doi: 10.1016/S0039-128X(97)00136-0
Numazawa, M., Yamada, K. 1999. Steroids 64, 320-327. doi: 10.1016/S0039-128X(98)00113-5
Oxford Diffraction, 2009. CrysAlis CCD and CrysAlis Red. Oxford Diffraction, Abingdo. Parsons, S., Flack, H. D., Wagner, T. 2013. Acta Cryst. B 69, 249-259. doi: 10.1107/S2052519213010014
Rigaku Oxford Diffraction, 2015.
Salvador, J. A. R., Carvalho, J. F. S., Neves, M. A. C., Silvestre, S. M., Leitao, A. J., Silva, M. M. C., Sa e Melo, M. L., 2013. Nat. Prod. Rep., 30, 324-374. doi:10.1039/c2np20082a
Sheldrick, G. M. 2015. Acta Crystallogr. A 71, 3–8. doi: 10.1107/S2053229614024218
Šoškić, V., Vujović, M., Stefanović, M. 1993. J. Serb. Chem. Soc. 58, 21-23.
Yadav, M. R., Barmade, M. A., Tamboli, R. S., Murumkar, P. R. 2015. Eur. J. Med. Chem. 105, 1-38. doi:10.1016/j.ejmech.2015.09.038
Zhang, S. J., Xu, F., Hu, W. X. 2010. J. Chem. Crystallogr. 40, 997-1001. doi: 10.1007/s10870-010-9782-y
Refbacks
- There are currently no refbacks.
ISSN 0354-4656 (print)
ISSN 2406-0879 (online)